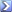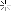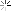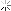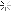oh and BTW they do release the ingredients. Just like anything that you find in the supermarket in Canada, it doesn't say the amount of each but here you are [GlaxoSmithKline supplies Canada with all of it's Flu Vaccines]
What are the ingredients of the H1N1 vaccine and adjuvant?
The H1N1 vaccine Arepanrix has been developed by GlaxoSmithKline using the egg-based production method. This means the vaccine viruses are grown in
eggs. This is true for the regular flu shot as well.
The manufacturer states the level of ovalbumin (egg protein) in the adjuvanted vaccine is generally below 5-10 ng/mL. The ovalbumin level is not yet available for the unadjuvanted version of the vaccine, which will be offered to pregnant women. (For egg and other allergy concerns, be sure to read:
H1N1 and Food Allergies.)
The vaccine has two components that are mixed together before the vaccine is given. The primary substance is the antigen drug substance for immunizing. It is administered with an adjuvant known as AS03.
The antigen contains: thimerosal, a mercury-based preservative used in the manufacturing of multidose vaccines, sodium chloride, disodium hydrogen phosphate, potassium dihydrogen phosphate, potassium chloride and water. As mentioned, there are traces of egg protein, and also of formaldehyde, sodium deoxycholate and sucrose.
The adjuvant AS03 is composed of: DL-alpha-tocopherol (vitamin E), squalene (shark liver oil) and polysorbate 80 (an emulsifier). In addition, its vial contains: sodium chloride, disodium hydrogen phosphate, potassium dihydrogen phosphate, potassium chloride and water. (For fish, soy allergy concerns, see
H1N1 and Food Allergies.)